Dry Cell vs. Wet Cell: Key Differences Explained
Advertisement
This article breaks down the key differences between dry cells and wet cells, two types of electrochemical cells used to provide power.
Introduction:
- Dry Cell: An electrochemical cell using an electrolyte with low moisture content. This design minimizes leakage, making dry cells ideal for portable devices.
- Wet Cell: An electrochemical cell containing a liquid electrolyte. Wet cells are commonly used in vehicles like cars and motorcycles as a power source.
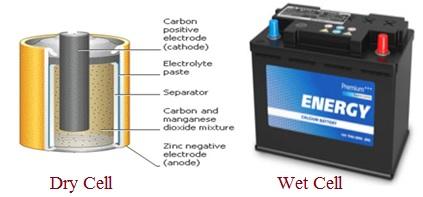
Figure 1: Depicts Dry Cell and Wet Cell.
The following table highlights the specific differences between these two types of cells:
| Specifications | Dry Cell | Wet Cell |
|---|---|---|
| Size | Small | Large |
| Electrolyte Type | Mostly solids | Liquids |
| Voltage Rating | 1.25V to 1.5V range | Higher voltages (12V, 15V, etc.) |
| Leakage | No leakage of chemicals | Corrosive chemicals can leak |
| Handling | Easy to handle | Difficult to handle |
| Cost | More expensive | Less expensive |
| Manufacturing | Difficult to manufacture | Easy to manufacture |
| Overcharging | Cannot withstand overcharging | Can withstand overcharging |
| Life | Shorter life | Longer life |
| Maintenance | No periodic maintenance required | Periodic maintenance required |
| Temperature Effect | Performance varies with temperature | Not very sensitive to temperature |
Advertisement
 RF
RF







